Classification of Air Pollutants
Introduction
To understand air pollutants, think of them as “unwanted guests” in the atmosphere.
They may differ in origin, behaviour, or chemical nature. Based on this, we classify them into primary, secondary, quantitative, and qualitative pollutants.
1. Primary Pollutants
These pollutants remain in the same form in which they were released.
They are persistent, meaning they continue to exist in the environment for long periods.
Examples:
- DDT
- Plastics
- Carbon monoxide (CO)
- Carbon dioxide (CO₂)
- Oxides of nitrogen (NOx)
- Oxides of sulphur (SOx)
These are harmful as they are, without needing further chemical transformation.
2. Secondary Pollutants
These are not emitted directly.
Instead, they form due to chemical reactions between primary pollutants.
Examples:
(a) Peroxyacetyl Nitrate (PAN)
Formed when NOx reacts with hydrocarbons.
(b) Ozone (O₃)
Ozone is a classic secondary pollutant. It forms in three ways:
- HCs/VOCs + NOx + Sunlight → Ozone
(This mechanism forms photochemical smog.) - When NO reacts with oxygen in air.
- Through processes associated with acid rain (NOx or SO₂ reacting with water).
Ozone is beneficial in the stratosphere (ozone layer), but harmful in the troposphere as a pollutant.
3. Quantitative Pollutants
These substances naturally exist in the atmosphere but become pollutants when their concentration crosses a safe threshold.
Examples:
- CO₂
- Nitrogen oxides
In essence: too much of a naturally occurring substance becomes harmful.
4. Qualitative Pollutants
These substances do not occur naturally and enter the environment only due to human activity.
Examples:
- DDT
- Fungicides
- Herbicides
These are entirely foreign to the natural system.
Particulate Pollutants
(Suspended Particulate Matter)
Particulate pollutants are solid or liquid particles suspended in the air—like dust, soot, smoke, and aerosols.
Size Matters (Literally)
- Range: 0.001 µm to 500 µm
- <10 µm → remain suspended, easily inhaled
- >10 µm → settle due to gravity
- <0.02 µm → form persistent aerosols that can remain airborne for very long
Major Sources:
Industries, vehicles, power plants, construction, refineries, railway yards, markets.
PM10 and PM2.5 — Why Are They Criteria Pollutants?
CPCB classifies PM10 and PM2.5 as major pollutants because they can enter deep into the respiratory system.
- PM10 (≤10 µm) → reaches upper respiratory tract
- PM2.5 (≤2.5 µm) → reaches deep into lungs, enters bloodstream
CPCB and global studies identify PM2.5 as the most harmful.
Health Impacts:
- Breathing difficulty
- Inflammation
- Irritation
- Pneumoconiosis
(lung disease caused by inhaling dust; involves coughing, inflammation, fibrosis)
Particulate Matter < 2.5 µm (PM2.5) and PM1
Arsenic and Nickel in PM
In the atmosphere, elements like arsenic and nickel exist as fine particles (<2 µm).
Nickel is released largely from:
→ petroleum processing
→ combustion in vehicles and power plants
Nickel compounds are carcinogenic.
Rising Attention Toward PM1
PM1 is <1 µm in size:
→ PM2.5 is about 30× thinner than human hair
→ PM1 is 70× thinner
Key Points About PM1:
- Hardly monitored anywhere
- WHO has no standards for PM1
- Around 40% of particulate matter may be as small as PM0.7, which is not monitored
- Can reach deepest parts of the respiratory system
- May penetrate the skin
- Contains high toxin content, including heavy metals
- Strongly associated with lung injury, gene damage, and cancer
Major Sources: Vehicular and industrial emissions.
Fly Ash
Fly ash is a major by-product of coal-based thermal power plants.
It can pollute both air and water, and also cause heavy metal contamination of soil and water.
Composition of Fly Ash
Fly ash is rich in oxides, mainly:
→ Silica (SiO₂)
→ Alumina
→ Iron oxides
→ Calcium and magnesium oxides
It also contains toxic heavy metals like:
→ Lead
→ Mercury
→ Cadmium
→ Arsenic
→ Cobalt
→ Copper
Uses of Fly Ash
Fly ash, though hazardous when unmanaged, is extremely useful when processed.
Major Uses:
- Replacement of cement (up to 35%) → cheaper construction
- Fly ash bricks → lightweight, durable
- Filling material for road embankments and concrete roads
- Reclamation of wastelands
- Filling up abandoned mines
- Enhancing soil water-holding capacity
(But deposition on leaves reduces photosynthesis.)
Policy Measures — Mandatory Utilisation
MoEF mandates the use of fly-ash-based products in:
→ construction projects
→ road embankments
→ landfilling work within 100 km of thermal plants
→ mine-filling within 50 km
Nanoparticles (NPs)
Nanoparticles have dimensions near 1 nanometre (10⁻⁹ m)—one-billionth of a meter.
Natural Sources:
Forest fires, volcanic eruptions, weathering, dust storms.
NPs:
- vary widely in size
- remain suspended for days
- can travel thousands of kilometres
Because of their tiny size, they have a very high surface-area-to-volume ratio, making them:
→ extremely reactive
→ capable of affecting climate, visibility, and health
Human-Made Nanoparticles
Produced during:
- industrial processes
- mechanical operations
- biomedical applications
- wastewater sludge handling
Nanotechnology is extremely beneficial (electronics, targeted drug delivery), but NP pollution is an emerging concern.
Environmental Effects of Nanoparticles
1. Dust Cloud Formation
NPs combine to form dust clouds.
Example: Asian Brown Cloud
These clouds:
- reduce sunlight reaching the surface
- deposit black carbon on Himalayan glaciers
- reduce albedo (reflectivity)
- increase glacier melting
2. Reaction with Hydroxyl Radicals (OH)
NPs quickly bind with hydroxyl radicals, reducing their concentration.
Why is this important?
Hydroxyl radicals are called the “detergents of the atmosphere” because they degrade pollutants like:
→ CO
→ CH₄
→ NOx
→ VOCs
→ HCFCs
So, fewer OH radicals mean reduced self-cleaning capacity of the atmosphere.
3. Ozone Depletion
NPs increase the formation of free radicals, such as chlorine radicals (Cl⁻), which destroy ozone molecules.
4. Stratospheric Cooling
Nanoparticles react with molecular hydrogen from hydrogen fuel cells.
Together, they move up to the stratosphere, forming ice-based stratospheric clouds.
These clouds:
- cool the stratosphere
- enhance ozone destruction
Thus, NPs indirectly contribute to global ozone depletion and climate imbalance.
Black Carbon (Soot)
Think of black carbon (BC) as tiny dark specks produced by incomplete combustion — from diesel engines, coal stoves, biomass burning, crop residue fires, and cookstoves. They are particulate matter (PM) and behave both as particles and as strong absorbers of sunlight.
Key points:
- Powerful sunlight absorber: BC absorbs sunlight much more effectively than CO₂, heating the air directly and changing local temperature structure.
- When it lands on snow/glaciers: it darkens the surface, lowers albedo (reflectivity), and causes faster melting — a direct threat to Himalayan glaciers.
- Short-lived climate pollutant: BC stays in the atmosphere only for days to weeks, unlike CO₂. That means cutting BC emissions gives rapid climate benefits regionally.
- Regional source mix (Indo-Gangetic Plain): roughly ~20% from biofuels, ~40% fossil fuels, ~40% biomass burning — so mitigation requires multi-sector interventions.
- Seasonal pattern: lower concentrations during monsoon (August) and highest in the dry pre-monsoon month (May).
Brown Carbon — the ‘imperfect combustion’ cousin
- Brown carbon is produced mainly by impure/low-temperature combustion of organic matter (biomass, some biofuels).
- It absorbs sunlight (less than BC) and contributes to regional haze and visibility reduction.
Carbon Monoxide (CO)
Nature & formation:
- Colourless, odorless, tasteless gas produced when carbon burns without enough oxygen (incomplete combustion).
- Natural sources: photochemical reactions, volcanoes, forest fires.
- Anthropogenic: vehicle exhausts, industrial processes (e.g., iron smelting), poor combustion in appliances.
Health effects:
- Binds with haemoglobin to form carboxyhaemoglobin, displacing oxygen — leading to dizziness, headache, unconsciousness; toxic above about 35 ppm for humans. CO poisoning is a common cause of fatal air poisoning.
Environmental role:
- Not a direct long-lived greenhouse gas, but it participates in atmospheric chemistry: it influences ground-level ozone formation and can indirectly increase methane concentrations by altering atmospheric oxidants.
Carbon Dioxide (CO₂)
Nature & sources:
- Colourless, odourless gas; heavier than air; released from volcanoes, dissolution of carbonate rocks, respiration, and combustion of fossil fuels.
Health:
- At very high concentrations CO₂ is an asphyxiant (≈7% can cause suffocation symptoms).
Environmental impacts:
- Major greenhouse gas driving global warming since the Industrial Revolution.
- Ocean acidification: CO₂ dissolves into seawater forming carbonic acid → lowers pH and harms marine life (coral reefs, shellfish).
Ozone (O₃)
Two “ozones” to remember:
Stratospheric ozone (the ‘good’ ozone)
- Located high up; absorbs harmful UV rays and protects life on Earth.
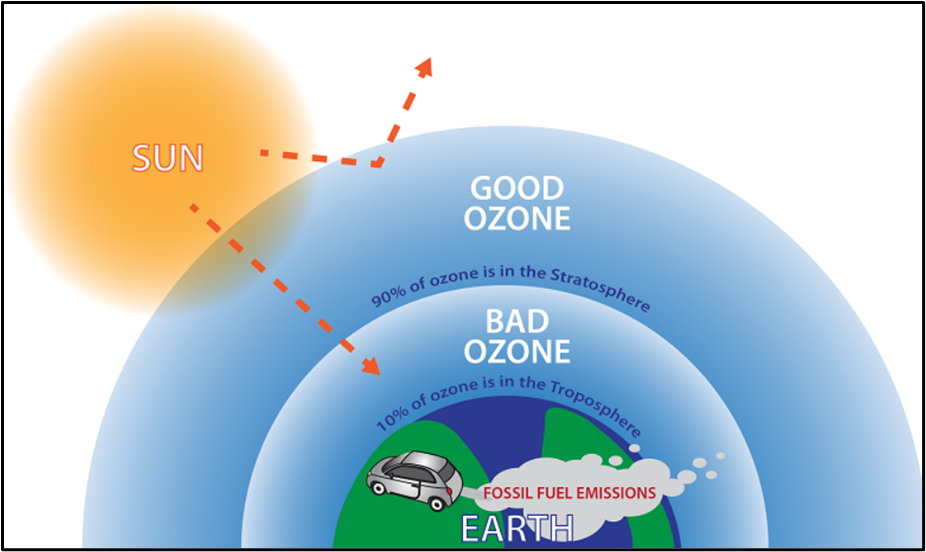
Tropospheric (ground-level) ozone (the ‘bad’ ozone)
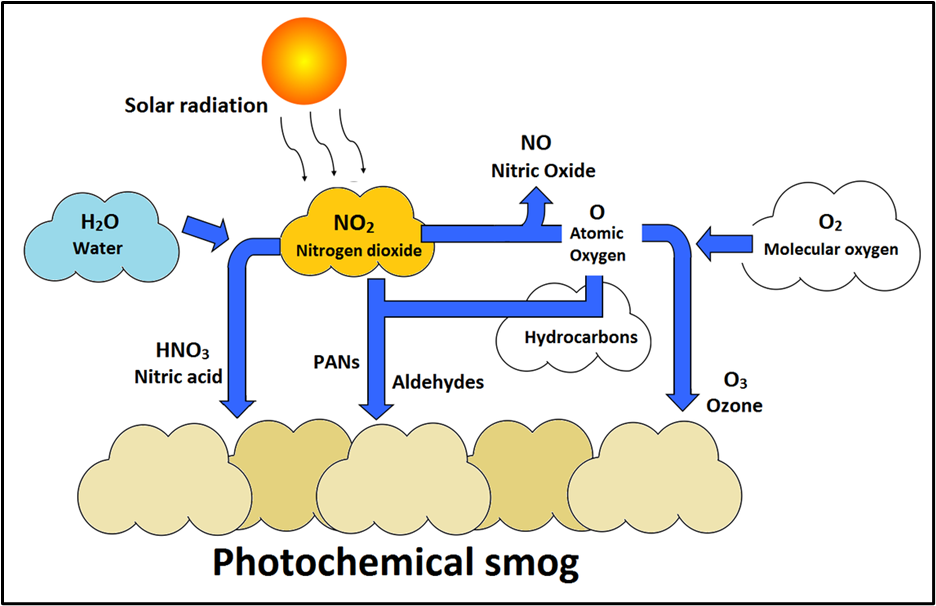
- Not emitted directly. It is a secondary pollutant formed by photochemical reactions among precursors like CO, NO₂, and VOCs in sunlight.
- Formation steps (photochemical smog chemistry simplified):
- CO + OH → HO₂
- VOCs + OH → RO₂ (peroxy radicals)
- HO₂/RO₂ oxidise NO to NO₂ + regenerate OH
- NO₂ under sunlight (photolysis) → O + O₂ → O₃
Health & environment:
- Causes eye irritation, reduces respiratory immunity, worsens asthma, and damages vegetation and crops (reduces yield and growth).
- Peaks on hot, sunny days and can be transported long distances by wind.
Stratospheric Ozone Depleting Substances (ODS)
These are human-made gases that release chlorine or bromine atoms in the stratosphere, which catalytically destroy ozone molecules.
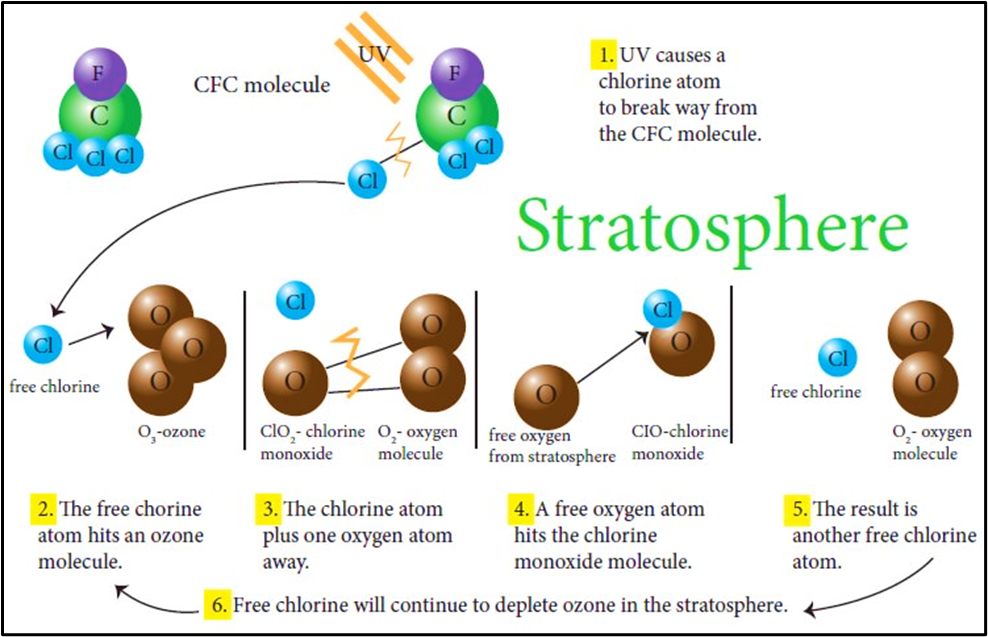
Important groups include:
- CFCs (chlorofluorocarbons)
- HCFCs (hydrochlorofluorocarbons)
- HBFCs (hydrobromofluorocarbons)
- Halons (used in fire extinguishers)
- Methyl bromide (fumigant)
- Carbon tetrachloride
- Methyl chloroform
Uses (historical and current): refrigerants, foam-blowing agents, solvents, dry-cleaning agents, aerosol propellants, fumigants, fire-fighting agents.
Important mechanisms:
- In the stratosphere, UV breaks these molecules → releases Cl· or Br· radicals → each radical can destroy many O₃ molecules through catalytic cycles.
Policy highlight:
- Montreal Protocol: an international treaty to phase out production of many ODS (including CFCs). This is the key policy instrument for ozone protection.
CFCs, HCFCs, HBFCs and Halons
- CFCs: highly stable, reach stratosphere easily, major ozone destroyers.
- Use heavily regulated and phased out under Montreal Protocol.
- Also potent greenhouse gases.
- HCFCs and HBFCs: interim substitutes for CFCs;
- less ozone-depleting but still GHGs and phased down as well.
- Halons: effective fire-extinguishing gases but ozone-depleting;
- production largely ended in developed countries in the 1990s.
Nitrogen Oxides (NOx)
Think of NOx as a family of gases made from nitrogen + oxygen, mainly created during high-temperature combustion.
How NOx Forms
- At normal temperatures, oxygen and nitrogen do not react.
- But inside internal combustion engines, coal-fired power plants, or anywhere combustion reaches very high temperatures, these gases easily combine to form Nitric Oxide (NO) and Nitrogen Dioxide (NO₂).
Natural sources also exist:
- Lightning
- Nitrogen-fixing microbes (enhanced by fertilisers and nitrogen-fixing plants)
Types of NOx
When we say NOx, we mostly mean:
- NO (Nitric Oxide) – colourless, odourless
- NO₂ (Nitrogen Dioxide) – reddish-brown, pungent
Other oxides include: NO₃, N₂O, N₂O₄, N₂O₅.
Important clarification for UPSC
- NO and NO₂ → contribute to global cooling
- N₂O (Nitrous Oxide) → greenhouse gas
Do NOT confuse these.
Effects of NOx on Health and Environment
- Reducing NOx is a major challenge, especially in biodiesel combustion.
- They aggravate asthma and reduce overall respiratory health.
- They help form:
- Acid rain (because they convert to nitric acid in moisture)
- Tropospheric ozone
- Photochemical smog (NOx + VOCs + sunlight)
Role in Global Cooling
NO and NO₂ help form OH radicals, which destroy methane, a potent greenhouse gas.
This creates a mild cooling effect.
Sulphur Dioxide (SO₂)
A toxic, pungent gas mainly released from burning sulphur-rich fuels.
Major Sources
- Thermal power plants (coal)
- Diesel fuel combustion
- Volcanic activity
- Metal smelting (copper, zinc, mercury ores like pyrite, sphalerite, cinnabar)
- Reactions involving H₂S
Health Effects
- Increases risk of stroke, heart disease, lung cancer, premature death.
- Major contributor to acid rain.
Global and Indian SO₂ Emission Trends (2024–2025)
- India remains the world’s largest anthropogenic SO₂ emitter, contributing ~20–22% of global emissions.
- Global SO₂ emissions have declined ~50% since 2005, mainly due to sharp reductions in China, Europe, and the US.
- India’s emissions stay high and persistent, driven by coal-based thermal power plants and slow FGD installation.
- China has achieved over 80% reduction in SO₂ emissions since 2006, no longer topping global rankings.
- Major Indian hotspots in Singrauli, Korba, Talcher–Jharsuguda, Neyveli–Chennai, Chandrapur, Kutch, Ramagundam.
Polyaromatic Hydrocarbons (PAHs)
These are toxic organic pollutants formed during incomplete combustion of:
- Coal, oil, petrol, wood
- Cigarettes
- High-temperature cooking (grilling, roasting)
Naphthalene, used in mothballs, is a common PAH.
Why are PAHs dangerous?
- Toxic, mutagenic, carcinogenic
- Highly lipid-soluble → bioaccumulate in body tissues
- Attach easily to PM2.5 and PM10, making particulates even more harmful.
Volatile Organic Compounds (VOCs)
These are carbon-based chemicals that evaporate quickly at room temperature.
Common examples:
- Benzene
- Formaldehyde
- Toluene
- Xylene
- Methylene chloride
- Ethylene glycol
Indoor sources
- Perfumes
- Hair spray
- Furniture polish
- Air fresheners
- Glues, wood preservatives
Health Effects
- Eye, nose, throat irritation
- Headaches, nausea
- Long-term: liver damage
Ethylene
- Widely used to make polyethylene (common plastic).
- Acts as a plant hormone → induces ripening of fruits.
- Low toxicity, but high exposure causes headache and drowsiness.
Ethylene oxide, however, is carcinogenic.
Formaldehyde
Found in:
- Plywood, particleboard, pressed wood
- Disinfectants and preservatives (e.g., mortuaries)
- Naturally produced during decomposition and combustion (e.g., tobacco smoke)
Benzene (Both VOC and PAH)
- Component of crude oil
- Found in cigarette smoke, forest fires, volcanoes
- Used to make plastics, resins, fibres, lubricants
- High octane number → used in petrol
Impact
- Forms ground-level ozone → contributes to smog
- Causes bone marrow failure, cancer
Related pollutants
- Toluene – in paint thinners, petrol octane booster
- Xylene – in printing, rubber and leather industries
- Styrene – used for polystyrene; note: 2020 Visakhapatnam gas leak
Minor Air Pollutants
Lead
Found in:
- Petrol (earlier), diesel, batteries, paints, dyes
Health effects:
- Damages kidney, liver, nervous system
- Reduces IQ in children
- Causes cumulative poisoning when mixed with food/water
Ammonia (NH₃)
A corrosive, pungent gas.
Sources:
- Decomposing organic matter
- Human and animal waste
- Fertilisers
- Livestock management
Effects:
- Irritates eyes, nose, throat
- Forms secondary PM2.5 (via ammonium salts)
- Causes nitrification and eutrophication in water bodies
Asbestos
Six naturally occurring fibrous silicate minerals:
- Chrysotile, crocidolite, amosite, anthophyllite, tremolite, actinolite
Health impacts:
- Lung cancer
- Mesothelioma
- Asbestosis (chronic lung disease)
Metallic Oxides
Oxides of iron, aluminium, zinc, magnesium, etc., especially from:
- Mining
- Metallurgical industries
Impact:
- Dust deposits on leaves → blocks photosynthesis
- Causes physiological and reproductive damage in plants
Biological Pollutants
Include:
- Pollen
- Mites
- Pet dander
- Fungal spores
- Bacteria
These commonly trigger asthma and allergic diseases.
Radon
- A naturally occurring radioactive gas from soil
- Accumulates indoors due to poor ventilation
- Can cause lung cancer
