Effects of Air Pollution
Think of air pollution as a series of insults to the atmosphere — some immediate and visible (smog, haze), some chemical and silent (acid rain, ozone chemistry) — but all of them connected by the same thread: human activity altering atmospheric chemistry and physics, and ecosystems paying the price.
1. Smog — Smoke + Fog
Smog is a visible, often harmful mixture of pollutants — soot, ozone, CO, SO₂, NO₂, VOCs — suspended in the air. Remember two classical types:
Sulphurous Smog (London smog)
- Source: burning sulphur-bearing fossil fuels (coal).
- Favoured by: damp weather and high suspended particulates.
- Appearance & effect: dense, choking grey smog; severe respiratory distress; historically associated with mortality peaks (London, 1952).
Photochemical Smog (Los Angeles smog)
- Source: high vehicular emissions in sunlight.
- Chemistry: NOx + VOCs + sunlight → O₃ (ozone) + PAN + other oxidants.
- Appearance: light brownish haze; causes reduced visibility, eye irritation, respiratory problems, plant damage.
- Peaks on hot sunny days.
UPSC tip: Sulphurous = coal + dampness; Photochemical = NOx + VOCs + sunlight → ozone & PAN.
Haze vs Smog
- Haze: dry-particle phenomenon — dust, smoke, dry aerosols obscure sky clarity (no condensation).
- Smog: haze plus condensation processes; chemically active and often more toxic.
- Inversion effect: Temperature inversion traps pollutants near ground → long persistence, worsened pollution (Delhi, Los Angeles, Beijing examples).
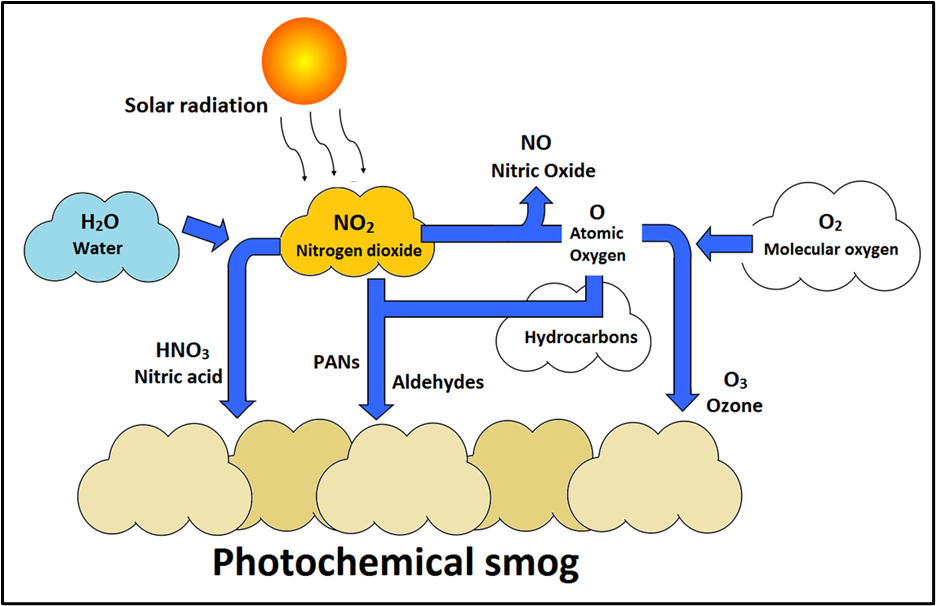
Photochemical Smog — simplified reaction chain
- NO + VOC → NO₂
- NO₂ + UV → NO + O (atomic oxygen)
- O + O₂ → O₃ (ozone)
- NO₂ + VOC → PAN (peroxyacetyl nitrate)
Net: NO + VOC + O₂ + UV → O₃, PAN, OH and other oxidants (these oxidants cause tissue & plant damage).
Remember: NOx + VOCs + Sunlight → Photochemical smog (O₃ + PAN).
Pollutant sources → environmental effects
- NO / NO₂ — Combustion, lightning, fires → decreased visibility (brownish), plant growth suppression, ozone & acid rain precursors.
- VOCs — fuel evaporation, incomplete combustion, solvents → eye/respiratory irritation, some carcinogenic; form ozone & secondary organics.
- O₃ (tropospheric) — photolysis product → reduces crop yields, retards plant growth, damages rubber & plastics, causes respiratory distress.
- PAN — formed from NO₂ + VOCs → eye irritation, highly phytotoxic.
2. Acid Rain
What is acid rain?
Any precipitation (rain, fog, mist, snow) with pH < 5.6 — more acidic than natural rainwater. (Remember: pH 7 neutral; each integer change = 10× change in H⁺.)
Main acidic gases
- SOx (SO₂, SO₃) — from fossil fuel burning, smelting, industry, volcanoes.
- NOx (NO, NO₂, N₂O) — combustion, lightning, biomass burning.
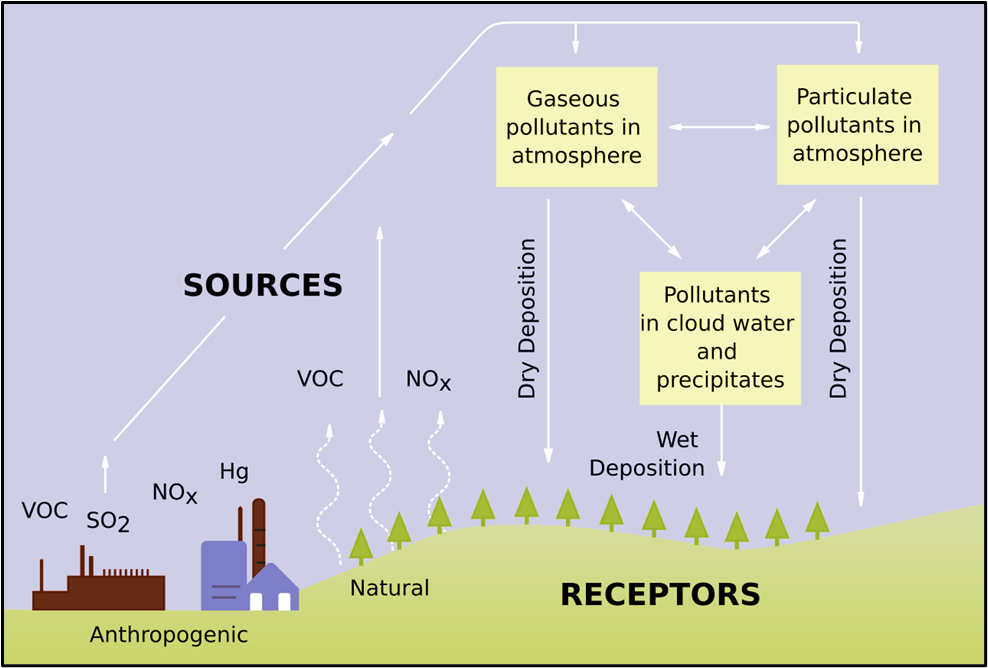
Basic chemistry (stepwise)
- Emission of SOx and NOx into the atmosphere.
- Some fall back by dry deposition (dust/smoke).
- Sunlight drives photo-oxidant formation (e.g., ozone) that oxidises SOx and NOx.
- Oxidation forms H₂SO₄ (sulphuric acid) and HNO₃ (nitric acid).
- These acids fall as wet deposition (rain, snow, fog) or adhere as dry deposition.
Wet vs Dry deposition
- Wet deposition: acids carried down by precipitation; affects vegetation, soil, water bodies.
- Dry deposition: acid gases/particles settle onto surfaces as dust/smoke; accounts for ~half of atmospheric acidity deposition.
Harmful effects of Acid Rain
Soil
- H⁺ ions exchange with nutrient cations (K⁺, Mg²⁺, Ca²⁺) → leaching → infertile soils.
- Alters microbial balance; may reduce decomposition rates and change nutrient cycles.
- Note (India): many Indian soils are alkaline and buffered, so immediate large-scale acidification is less pronounced in many regions — but local effects exist.
Humans
- Reduced visibility, foul smells, skin and respiratory irritation; long-term exposure linked to chronic bronchitis, emphysema, and increased mortality.
Aquatic life
- Low pH kills eggs/gametes of fish and amphibians → ecosystem disruption.
- Acidic waters dissolve metals from sediments/soil → toxic metal release (Al, Pb, Hg) → bioaccumulation and food-web impacts.
Terrestrial plants
- Damage to leaf cuticle → reduced photosynthesis → decreased growth and yields.
- Soil metal mobilization harms root systems and soil biota.
Microorganisms
- pH shifts alter microbial community composition (more fungi in acidic conditions) → slows decomposition, affects nutrient cycling.
Buildings & Monuments
- Limestone & marble (calcium carbonate) react with acids → surface dissolution and flaking (e.g., marble cancer on monuments like the Taj Mahal).
- Paints, metals, and infrastructure corrode faster.
Geographic patterns & India context
- Globally concentrated in industrialised belts of the Northern Hemisphere (Scandinavia, NE US, NW Europe, Japan, Canada).
- In India: first reported acid rain incident in Bombay (1974); occurrences now in many metros. Low pH soils reported in NE India, coastal Karnataka & Kerala, parts of Odisha, West Bengal, Bihar.
- India has significant SO₂ hotspots around coal power clusters — relevant to acid deposition risks.
Control and mitigation measures
- Fuel switching/cleaner fuels: use low-sulphur fuels or washed coal in thermal plants.
- Flue gas desulphurisation (FGD): scrubbers to remove SO₂ at source.
- Emission norms & enforcement: stricter standards for power plants, industries, vehicles.
- Buffering (remedial): liming acidic water bodies/soils using calcium carbonate or lime (CaO) to neutralise acidity — a treatment, not prevention.
- Renewables & efficiency: reduce fossil fuel combustion overall.
- Monitoring & mapping: identify hotspots and prioritise action
3. Ocean Acidification
Ocean acidification is often described as the other CO₂ problem because it is not just warming the oceans — it is changing their chemistry.
What is Happening?
The oceans are naturally alkaline (pH ≈ 8.1).
When CO₂ dissolves in seawater, that pH gradually drops — becoming less alkaline and more acidic.
This entire process is known as ocean acidification.
Main Driver
- CO₂ uptake by oceans (about 30–40% of human-emitted CO₂).
- Therefore, reducing CO₂ and CO emissions is the only permanent solution.
Mechanism — How CO₂ Lowers Ocean pH
- CO₂ dissolves in water → forms carbonic acid (H₂CO₃).
- Carbonic acid dissociates → forms bicarbonate ions (HCO₃⁻) + hydronium ions (H⁺).
- More H⁺ → lower pH (higher acidity).
The key point:
An increase in CO₂ causes an increase in H⁺ concentration → ocean pH decreases.
Other Contributors to Ocean Acidification
1. Eutrophication
- Excess nutrients → plankton blooms.
- When blooms die and sink, bacteria decompose them → oxygen drops and CO₂ rises → local acidification.
2. Melting Arctic Ice
This accelerates acidification in 3 ways:
- Meltwater exposes deeper CO₂-poor water to atmospheric CO₂ → more absorption.
- Dilution reduces carbonate ion concentration → decreases buffering capacity.
- Meltwater is lighter → prevents mixing with deeper waters → CO₂ accumulates near the surface.
Effects of Ocean Acidification
1. Reduced Buffering Capacity
Oceans have historically acted as Earth’s “CO₂ sponge.”
But when acidity rises too fast, natural buffering fails.
Impacts include:
- Stress on metabolic and immune systems of marine organisms.
- Greater vulnerability to disease and climate stress.
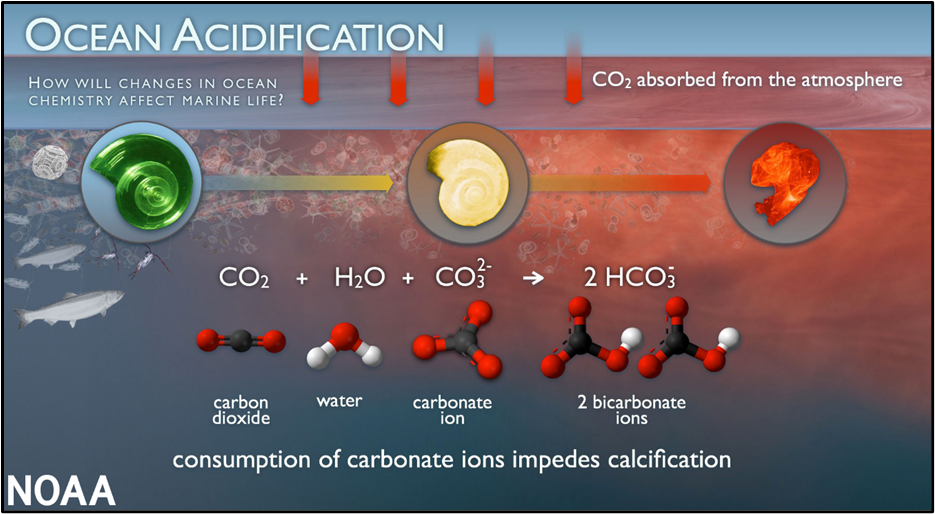
2. Threat to Marine Calcifiers (Corals, Shell-Forming Plankton, Molluscs)
Marine calcifying organisms require carbonate ions (CO₃²⁻) to build calcium carbonate (CaCO₃) shells and skeletons.
But acidification causes:
- A drop in carbonate ions
- More bicarbonate, less carbonate
This leads to:
- Weak coral skeletons
- Reduced plankton productivity
- Threats to entire fisheries, because plankton form the base of marine food webs
This even worsens coral bleaching, already aggravated by warming.
3. Impact on Cloud Formation
Marine clouds are partly regulated by aerosols derived from dimethylsulfide (DMS) produced by phytoplankton.
Steps:
- Phytoplankton produce DMS.
- DMS enters the atmosphere and forms sulphuric acid aerosols.
- These act as cloud condensation nuclei (CCN) → more clouds → more sunlight reflected → cooling effect.
But acidified oceans ➡️ less DMS produced ➡️ ↓ fewer aerosols ➡️ ↓ cloud formation ➡️ ↑ more warming.
Thus, ocean acidification can indirectly reduce cloud cover and intensify global warming.
4. Aerosols and Monsoon Rainfall
What are Aerosols?
Tiny solid or liquid particles suspended in the air.
They may be:
- Natural: fog, dust, volcanic ash, sea spray
- Anthropogenic: soot, industrial pollutants, smoke
Aerosols help form cloud droplets and ice crystals.
Most are located in the lower troposphere (<1.5 km), but volcanic aerosols can reach the stratosphere.
Aerosols and Monsoon Behaviour
Aerosols have complex and uncertain effects because they both:
➡️Scatter sunlight
➡️Absorb sunlight
➡️Modify cloud properties
➡️Change atmospheric heating patterns
Why are monsoons affected?
The Indian monsoon relies on a strong land–sea temperature gradient.
Aerosols reduce sunlight reaching the land (due to scattering and smog), causing:
➡️ Cooling of land
➡️ Weakened temperature gradient
➡️ Weaker monsoon circulation
Hence, monsoon rainfall becomes suppressed or erratic.
However, aerosols can also enhance rainfall in specific regions, especially:
➡️ Himalayan foothills
➡️ Urban areas with high aerosol loading
This causes regional rainfall disparities (urban flooding + rural drought).
Aerosols and High Rainfall Events in the Himalayan Foothills
Scientists have observed:
- Increase in short-duration, heavy rainfall events.
- Cause: black carbon + dust aerosols + orographic uplift.
- Result: Intense cloud formation leading to localised heavy rainfall.
ATAL — Asian Tropopause Aerosol Layer
ATAL is a monsoon-season aerosol layer sitting in the Upper Troposphere–Lower Stratosphere (UTLS) at 12–18 km altitude.
Formation
- Strong monsoon convection lifts aerosols from the surface into UTLS.
- Aerosols come from:
- Black carbon (North India, East China — accentuated during El Niño)
- Sulphates (East Asia)
- Organic aerosols, nitrates, dust
Impact of ATAL
- Increased sulphate aerosols in UTLS → strong cooling effect (scatter sunlight).
- Suppresses monsoon rainfall.
- During El Niño:
→ normal monsoon decreases
→ aerosols worsen this by another 17% reduction over central India.
Aerosols and Regional Rainfall Distribution
- Urban areas → more high-rainfall events (due to aerosol-induced microphysical cloud changes).
- Rural areas → rainfall deficit.
This creates spatial inequalities in precipitation patterns.
5. Ozone Depletion
The ozone layer in the stratosphere acts like Earth’s sunscreen, absorbing harmful UV-B radiation.
However, over the past several decades, a steady decline has been observed, especially over polar regions.
This is not uniform thinning — it is chemically driven destruction linked to halogen-containing gases (CFCs, HCFCs, halons, carbon tetrachloride, etc.).
Why is the Ozone Hole most prominent over Antarctica?
1. Strong Polar Vortex
- Antarctica experiences a very strong circumpolar vortex — a giant cyclone-like circulation.
- This isolates the polar stratosphere and traps chemical species within it.
2. Extremely Low Temperatures
- Winters in the Antarctic stratosphere are so cold that Polar Stratospheric Clouds (PSCs) form.
- PSCs accelerate ozone destruction by activating halogen radicals.
Result:
A dramatic drop in ozone concentration during Antarctic spring (September–November), producing the infamous Antarctic Ozone Hole.
The Arctic also gets ozone depletion, but:
- its polar vortex is weaker,
- stratospheric temperatures are higher,
→ so ozone loss is smaller and less consistent.
Role of Halocarbons and Halogen Radicals
Halocarbons = hydrocarbons where hydrogen atoms are replaced by halogens (chlorine, fluorine, bromine, iodine).
Examples: CFCs, HCFCs, halons, carbon tetrachloride, trichloroethane.
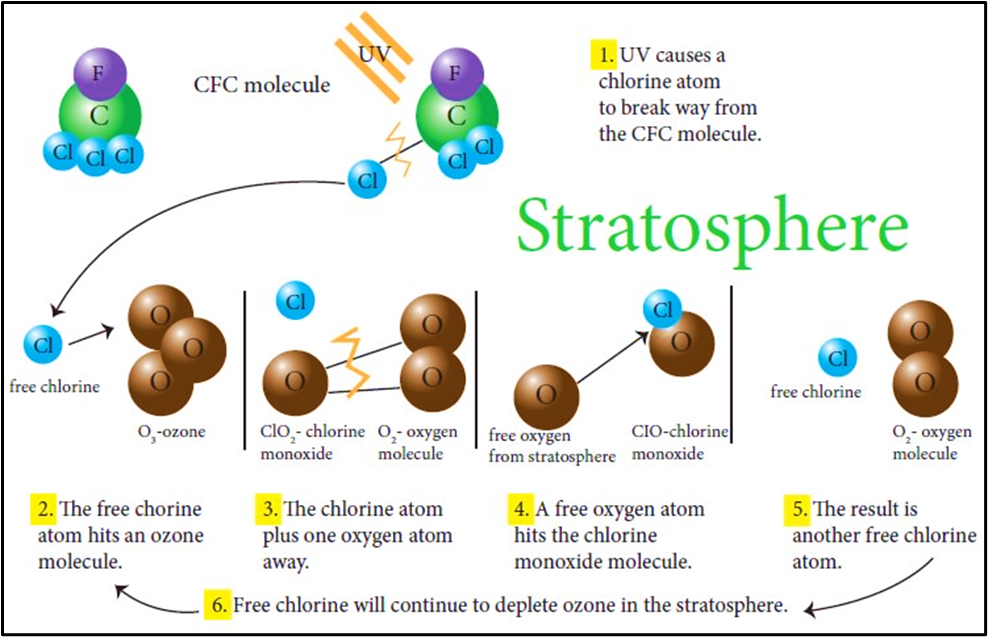
How they destroy ozone
- UV light photodissociates these compounds in the stratosphere.
- Free chlorine atoms (Cl·) are released.
- Each Cl atom can destroy thousands of ozone molecules through catalytic cycles.
This is why even small concentrations of CFCs cause massive ozone loss.
POLAR STRATOSPHERIC CLOUDS (PSCs) — The Catalysts
PSCs form at 12–22 km altitude during the dark, cold polar winter.
They consist of:
➡️ water vapour,
➡️ nitric acid,
➡️ sulphuric acid.
Why PSCs accelerate ozone depletion
PSCs convert halogen reservoir compounds into highly reactive radicals (like Cl· and ClO·).
These radicals then rapidly destroy ozone once sunlight returns in spring.
Thus, PSCs are essential for the formation of the Antarctic Ozone Hole.
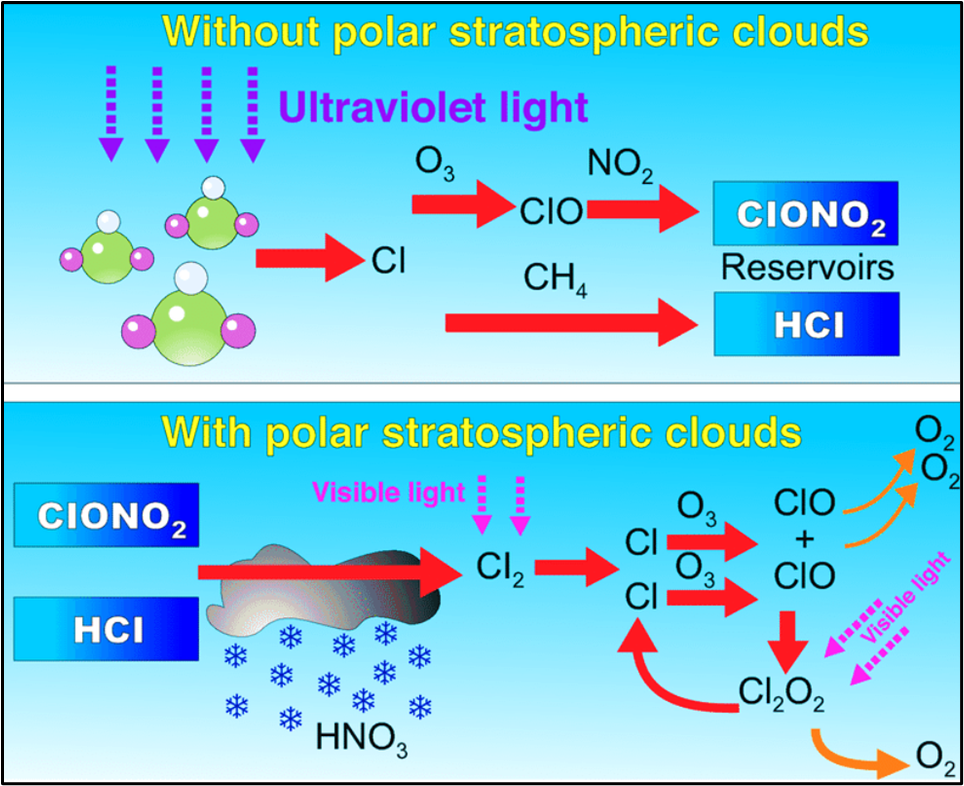
Without PSCs, chlorine stays inactive. With PSCs, chlorine becomes active and destroys ozone rapidly.
Role of Nanoparticles (NPs)
OH radicals are the key “detergents of the atmosphere”—they help break down many ozone-depleting substances.
Nanoparticles:
- react with OH,
- reduce OH concentration,
→ slowing the breakdown of ozone-depleting substances.
This means NPs indirectly help maintain higher levels of ODS in the stratosphere, thereby prolonging ozone depletion.
Tropical Ozone Hole — A New Concern
Recently, scientists discovered a large, year-round ozone hole over the tropics, present since the 1980s but unnoticed earlier.
Features:
- Exists throughout the year, unlike the Antarctic hole (seasonal).
- Approximately 7 times larger in area than the Antarctic ozone hole.
- Has similar depth (extent of depletion).
This has major implications because:
- the tropics contain ~50% of global population,
- the tropical ozone layer shields the most intense UV radiation zone.
Mesosphere’s Role in Antarctic Ozone Depletion
Within the polar vortex, air from the mesosphere descends into the stratosphere.
This mesospheric air contains nitrogen oxides (NOx) generated via breakdown of nitric acid.
NOx reacts with ozone → destroys O₃.
Thus PSCs (halogen radicals) + Mesospheric NOx together intensify Antarctic ozone loss.
6. Health Impacts of Air Pollution
Air pollution is not just an environmental issue — it is a public health crisis.
The State of Global Air (SoGA) 2025 Report
Released by:
→ Health Effects Institute (HEI) and
→ Institute for Health Metrics and Evaluation (IHME), United States.
Key findings about air pollution in India
- Mortality: Around 2 million deaths in 2023 linked to air pollution, a 43% rise since 2000, and 52% of the global share.
- Ozone Pollution: India has third highest exposure to ozone pollution.
- Exposure: 75% population exposed to PM2.5 above WHO limits.
- Pradhan Mantri Ujjwala Yojana (PMUY): Modelling studies suggest that transitioning all PMUY households to exclusive LPG use would avert more than 150,000 deaths annually
Global Picture:
- 36% of the world’s population is exposed to levels of PM2.5 pollution above the least stringent interim target of 35 μg/m3.
- About 1/3 – almost 2.6 billion people – are exposed to pollution from burning solid fuels for cooking at home.
- 95% of air pollution attributable deaths in adults over the age of 60 are due to noncommunicable diseases like COPD, dementia, diabetes, heart disease, and lung disease.
Occupational Health Hazards from Air Pollutants
Certain industries expose workers to harmful chemicals and particulates.
Carcinogenic Agents
- Benzene
- Chromium
- Nitrosamines
- Asbestos
Cancers linked: lung, bladder, skin, mesothelioma, liver.
Occupational Lung Diseases
- Silicosis
- Inhalation of silica dust
- Common among miners, pottery, ceramics workers
- First reported in India (Kolar Gold Fields, 1947)
- Pneumoconiosis
- Broad category: lung disease due to dust inhalation
- Coal dust → Black Lung Disease (Anthracosis)
- Byssinosis
- Among textile workers
- Caused by cotton dust
- Leads to chronic breathing problems
- Asbestosis
- Due to asbestos fibre inhalation
- Causes lung scarring and cancers
Black Lung Disease: lungs turn black from coal dust accumulation over years.
