Pyrites
(Iron Disulfide – FeS₂)
Imagine you see a shiny, yellowish mineral sparkling in the rocks and you mistake it for gold — only to find out it’s worthless. This very experience gave pyrite its common nickname: “Fool’s Gold.” But don’t be fooled 😄 — pyrite has immense industrial value, especially as a source of sulphur, which plays a central role in the chemical industry.
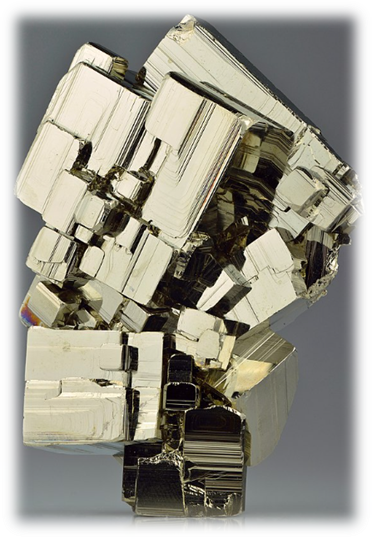
Physical Properties & Industrial Importance
- Chemical Composition: Iron disulfide (FeS₂)
- Colour: Brass-yellow (gold-like)
- Streak: Brownish-black
- Lustre: Metallic
- Hardness: Harder than gold, brittle
- Historical Use: Prehistoric humans used pyrite to start fires by striking it against flint or other hard stones, producing sparks.
✅ Modern Applications:
- Main source of sulphur for sulphuric acid production.
- Acts as a soil conditioner in agriculture.
- Also includes related minerals like:
- Marcasite (in sedimentary/metasedimentary rocks)
- Pyrrhotite (often with nickel, found in magmatic/metasomatic settings)
Formation of Pyrites: How Nature Makes It
Pyrite is geologically versatile, meaning it can form under multiple conditions and settings:
- Hydrothermal Activity
- Superheated fluids circulate through cracks and cool down, precipitating pyrite in veins and cavities.
- Bacterial Activity
- In anoxic sedimentary environments (e.g. swamps, ocean floors), sulfate-reducing bacteria react with organic matter to form sulphide, which then bonds with iron to form pyrite.
✅ This is a biogenic process – linking biology with geology.
- In anoxic sedimentary environments (e.g. swamps, ocean floors), sulfate-reducing bacteria react with organic matter to form sulphide, which then bonds with iron to form pyrite.
- Metamorphism
- During regional metamorphism, iron-bearing minerals transform under heat and pressure into pyrite, often giving rise to coarse-grained crystals.
- Weathering and Oxidation
- At or near the surface, pyrite oxidizes to form secondary minerals (like limonite, goethite, or sulfuric acid in some cases) while still preserving its original crystalline shape (a process called pseudomorphing).
Distribution of Pyrites in the World
Globally, pyrite isn’t just common — it’s geologically widespread, often forming as by-products in mining areas for gold, copper, or coal.
🌐 Major Occurrences
| Region | Notable Locations |
|---|---|
| Europe | Iberian Pyrite Belt (Spain, Portugal), Rio Tinto Mine (Spain) |
| North America | Appalachian Mountains (USA) |
| Russia | Ural Mountains |
| Asia | China – major producer |
🌐 Leading Producers:
- China
- Peru
- Spain
- Russia
- USA
Distribution of Pyrites in India
Though not as prominent as coal or iron, pyrite has strategic value in India for its sulphur content, and hence is tied closely to the chemical fertilizer and phosphatic industry.
📍 State-wise Distribution
| State | Notable Areas | Remarks |
|---|---|---|
| Rajasthan | Son Valley (largest), Zawar (Udaipur), Agucha (Bhilwara) | 94% of India’s reserves |
| Karnataka | Chitradurga, Uttar Kannada | Moderate deposits |
| Assam | Found in coal and shale within coalfields | Pyritous nature of coal |
| Bihar | Smaller occurrences | Legacy deposits |
📦 Reserve Status:
- As per 2020 data, India’s total pyrite reserves: 25.7 million tonnes (MT)
- Rajasthan alone accounts for over 94%
🏭 Industrial Context in India
- The government once supported pyrite mining through Pyrites Phosphates and Chemicals Ltd (PPCL):
- Two plants were set up in Rajasthan and Bihar.
- A phosphorite division operated in Dehradun.
- However, like many public sector units, PPCL struggled and is now defunct.
✅ Why did India lose interest in pyrites?
Because modern sulphur requirements are now largely met as by-products from petroleum refining and natural gas processing, making direct mining of pyrites less economical.
