Lithium
Let’s begin with a visual: imagine a shiny, silver-white metal so light that it would float on water if it didn’t react explosively with it. That’s Lithium, the lightest metal known to humanity — with a density of just 0.534 g/cm³, which is half the density of water.

But don’t be fooled by its beauty or softness — this metal is highly reactive and flammable. In fact, if exposed to air, it quickly loses its shine, turning grey and then black due to corrosion. In fact, if you see the above image of a freshly cut sample of lithium, it shows some oxides already formed over it. Now, to prev6ent this, it’s stored carefully in mineral oils like kerosene or diesel.
- It is a non-ferrous metal — meaning it doesn’t contain iron.
- It’s an excellent conductor of heat and electricity.
- When it comes into contact with water, it reacts violently, producing hydrogen gas and lithium hydroxide.
Because of this fiery nature and its growing importance in modern technology, Lithium has earned the nickname: “White Gold” — especially due to its demand in rechargeable batteries.
Occurrence of Lithium in Nature
Now, here’s something interesting: Lithium never occurs in free form in nature. You’ll never find pure lithium lying around like gold nuggets. Instead, it is found within certain rocks and salty waters, mainly in two forms:
a) Pegmatitic Minerals (Hard Rock Deposits)
Lithium often occurs in a rock called pegmatite — which is a coarse-grained igneous rock. Think of this rock as the final crystallized product of magma, often carrying rare minerals and large crystals.
So, lithium in this form is found deep within hard rock deposits — commonly mined in countries like Australia, China, and the USA.
b) Brine Deposits (Salt Lakes)
Lithium is also found dissolved in saltwater — especially in brines, which are highly concentrated salt solutions.
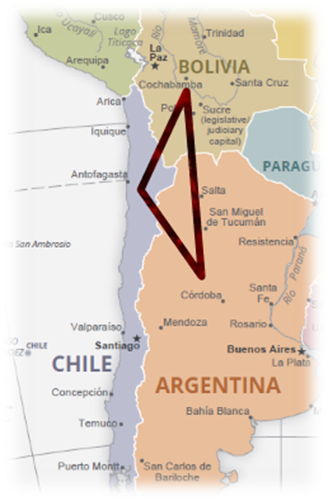
These are most common in underground salty lakes or Salars, prominently found in Chile, Argentina, and Bolivia — collectively known as the “Lithium Triangle”, a region that holds 54% of the world’s lithium reserves.
Extraction and Applications
The extraction process is quite technical. Pure lithium metal is separated electrolytically from a molten mix of lithium chloride and potassium chloride.
And once extracted, its applications are diverse and crucial in the modern world:
🔋 Rechargeable Batteries – The crown jewel of lithium use:
- Smartphones, laptops, and especially electric vehicles (EVs) rely heavily on lithium-ion batteries.
🧠 Non-rechargeable Batteries
- Used in critical medical devices like pacemakers, and small gadgets like toys.
⚙️ Light Alloys
- Forms lightweight and strong alloys with aluminium and magnesium — valuable for aircraft and automobile industries.
🧪 Other Applications:
- Lithium carbonate: Used in treating manic depression (bipolar disorder).
- Lithium stearate: Used as a lubricant.
- Lithium oxide: For making special glasses.
- Lithium chloride: Used in air-conditioning systems.
- Lithium hydride: Used for hydrogen storage.
Lithium in India: Potential but Untapped
India has huge potential but is yet to fully utilise it.
- Estimated reserves: 5.9 million tonnes of Lithium Carbonate Equivalent (LCE).
- Found mostly in hard rock pegmatites.
- Major sites:
- Salal-Batote-Reasi belt in Jammu & Kashmir.
- Revant Hill, Degana, in Nagaur district, Rajasthan.
🛑 No commercial production of lithium has begun in India yet. We still rely on imports.
Lithium Across the World
Let’s look at the global geography of lithium:
a) Lithium Triangle (Chile, Argentina, Bolivia)
- These salt-flat regions are rich in brine-based lithium.
- Hold over half the world’s reserves.
- Remember this as ABC triangle 😊
b) Salton Sea, California (USA)
- A recently discovered massive deposit under the Salton Sea, estimated to hold 18 million tonnes of lithium.
c) McDermitt Caldera (USA)
- A supervolcano-turned-caldera, potentially holding 20–40 million tonnes — the largest known concentration in the world.
🌋 Caldera: A large depression formed after a volcanic eruption and collapse.
💥 Supervolcano: A volcano capable of erupting >1,000 km³ of material (VEI-8 on the Volcano Explosivity Index).
This deposit could make the US self-sufficient, reducing dependence on China, which dominates the lithium battery supply chain.
World Data (2024)
Let’s conclude with some numbers:
🌍 World Reserves of Lithium (in million tonnes – MT):
| Country | Reserves |
| Chile | 9.3 MT |
| Australia | 7.0 MT |
| Argentina | 4.0 MT |
| China | 3.0 MT |
| World | 30 MT |
🏭 World Production of Lithium (in thousand tonnes – TT):
| Country | Production |
| Australia | 88 TT (37%) |
| Chile | 49 TT (20%) |
| China | 41 TT (17%) |
| Zimbabwe | 22 TT (9%) |
| World | 240 TT |
In Summary
- Lithium is a critical mineral — central to the global green energy transition.
- Though India has reserves, we lack commercial production.
- Global geopolitics around lithium is intensifying, as countries strive for energy security in the age of electric vehicles and battery storage.
This is not just about a metal — it’s about strategic resources, sustainable development, and the future of technology.
